Background
MBL is a precursor to chronic lymphocytic leukemia (CLL) and is subclassified into low-count (LC) MBL (absolute B-cell count<0.5x109/L) and high-count (HC) MBL (absolute B-cell count between 0.5 and 5x109/L). We previously reported that a polygenic risk score (PRS) based on a weighted average of 41 CLL-susceptibility variants was associated with risk of both MBL and CLL among a cohort of individuals from CLL families. Here we evaluate this PRS in an independent cohort of MBL and CLL individuals of European ancestry (EA), all of whom were ascertained agnostic to CLL family-history status. We also evaluate the PRS by MBL subtype (LC/HC), and in African American (AA) CLL cases and controls.
Methods
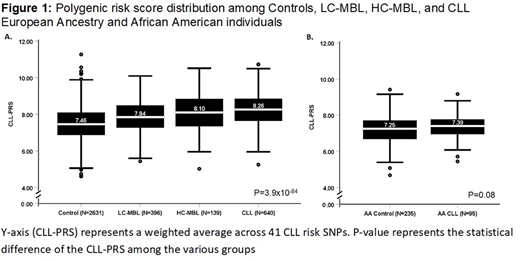
We genotyped 535 EA MBLs (139 HC-MBL, 396 LC-MBLs), 735 CLLs (640 EA, 95 AA), and 2,866 controls (2,631 EA, 235 AA) from the Mayo Clinic CLL Resource, Duke University, and Weill Cornell Medical College. We computed the CLL-PRS for each individual and used logistic regression to estimate odds ratios (OR) and 95% confidence intervals, adjusting for age and sex. To assess discriminatory accuracy, we computed the c-statistic. Among EA individuals, we calculated a trend test among LC-MBL, HC-MBL, and CLL risk using the P-value for heterogeneity from a polytomous logistic regression analysis. Moreover, we plotted a boxplot for the PRS among controls, LC-MBL, HC-MBL, and EA CLL, as well as for AA CLL cases and controls, and tested the statistical difference using the Kruskal Wallis test and Mann-Whitney test, respectively.
Results
We found a significant association of PRS with overall MBL risk (OR=1.87, P=1.1x10-28) with good discrimination (c-statistic=0.72). Significant associations were also found for LC-MBL (OR=1.75, P=7.5x10-19, c-statistic=0.72), HC-MBL (OR=2.22, P=1.4x10-17, c-statistic=0.74), and CLL of EA (OR=2.60, P=1.2x10-62, c-statistic=0.78), with a significant difference among these cohorts (Figure 1.A) and a significant positive trend across these cohorts (Pheterogeneity=8.4x10-6). Although we observed a 33% increased risk of CLL in AA (c-statistic=0.57), the PRS was borderline significant (P=0.07, Figure 1.B).
Conclusion
The CLL-PRS is a strong prediction-tool for risk of CLL and MBL among individuals of EA. Future studies are needed to improve the PRS for AAs including performing GWAS of AA in order to identify CLL-susceptibility SNPs that are more representative within known CLL loci and to discover novel CLL loci that are unique for AAs.
Parikh:GlaxoSmithKline: Honoraria; Janssen: Honoraria, Research Funding; Ascentage Pharma: Research Funding; AbbVie: Honoraria, Research Funding; Merck: Research Funding; TG Therapeutics: Research Funding; Genentech: Honoraria; Pharmacyclics: Honoraria, Research Funding; MorphoSys: Research Funding; AstraZeneca: Honoraria, Research Funding; Verastem Oncology: Honoraria. Braggio:DASA: Consultancy; Bayer: Other: Stock Owner; Acerta Pharma: Research Funding. Brander:Genentech: Consultancy, Honoraria, Other, Research Funding; Juno/Celgene/BMS: Other, Research Funding; MEI Pharma: Other, Research Funding; Ascentage: Other, Research Funding; ArQule: Consultancy, Other, Research Funding; NCCN: Other; Teva: Consultancy, Honoraria; Tolero: Research Funding; AstraZeneca: Consultancy, Honoraria, Other, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other, Research Funding; Pfizer: Consultancy, Other; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Novartis: Consultancy, Other; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Tolero: Research Funding; Teva: Consultancy, Honoraria; DTRM: Other, Research Funding; BeiGene: Other, Research Funding; Novartis: Consultancy, Other; NCCN: Other; Verastem: Consultancy, Honoraria, Other, Research Funding. Cerhan:NanoString: Research Funding; BMS/Celgene: Research Funding. Kay:Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Agios Pharma: Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma: Research Funding; Juno Theraputics: Membership on an entity's Board of Directors or advisory committees; Morpho-sys: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees; Cytomx: Membership on an entity's Board of Directors or advisory committees; Tolero Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squib: Membership on an entity's Board of Directors or advisory committees, Research Funding; MEI Pharma: Research Funding; Abbvie: Research Funding; Oncotracker: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees. Furman:Acerta: Consultancy; AstraZeneca: Consultancy, Research Funding; Beigene: Consultancy; Abbvie: Consultancy; Pharmacyclics: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy, Research Funding; Verastem: Consultancy; Incyte: Consultancy; Genentech: Consultancy; Janssen: Consultancy, Speakers Bureau; Loxo Oncology: Consultancy; Oncotarget: Consultancy. Shanafelt:Mayo Clinic: Patents & Royalties: and other intellectual property; Genentech, Pharmacyclics LLC, an AbbVie Company, AbbVie, GlaxoSmithKline, and Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal